
Global ASCVD Trends and Emerging Strategies

Program Director
Peter Libby, MD

Program Director
Raul D Santos, MD, MSc, PhD
The IAS has developed the worldwide effort aimed at health care professionals to comprehensively address the matter of LDL-C attributable risk in the prevention of Major Adverse Cardiology Events (MACE) and mortality This will be accomplished through a series of nine (9) videos presented by some of the most recognizable and prominent leaders in cardiology and lipid medicine from across the globe.
Importantly, the videos will emphasize the translation of current guidelines and evidence into practice approaches that heighten both clinical and patient success. Every video, based on real-world issues and evidence, will focus on implementing practical physician and patient strategies.
The videos are presented in English, but we have added subtitles in four (4) additional languages: Arabic, Spanish, Portuguese and Chinese.
Click on the button of your preferred language below.

Pre-Activty Information
 This activity is designed for an audience of Global healthcare professionals, including endocrinologists, diabetologists, lipidologists, cardiologists, and primary care clinicians (PCPs).
This activity is designed for an audience of Global healthcare professionals, including endocrinologists, diabetologists, lipidologists, cardiologists, and primary care clinicians (PCPs).

Upon completion of the educational activity, participants should be able to discuss ongoing therapeutic developments in the field. Discussion to include: PCSCK9 inhibitors for pediatric patients, ANGPTL3 inhibitors, Lp(a) therapies, and other novel approaches.
- Video 1 will present the evidence from epidemiological, Mendelian Randomization and interventional studies.
- Video 2 will discuss the lack of cholesterol goal attainment in clinical practice using evidence and data from EUROASPIRE, ICLPS, Da Vinci et al.
- Video 3 will discuss the available evidence for intensive LDL-C lowering strategies.
- Video 4 will discuss the evidence of safety related to intensive LDL-C reduction with respect to the issues of cognition, hemorrhagic stroke, liver, muscle, glucose, hormones, and vitamins.
- Video 5 will discuss the efficacy and safety of monoclonal PCSK9 inhibitors.
- Video 6 will discuss the impact of PCSK9 inhibition on specific groups of patients that are at greater risk and will explore how to increase have greater absolute benefit and cost effectiveness of therapy after: recent MI, demonstrated elevated atherosclerosis burden, diabetics, higher Lp(a), and CKD, as seen in FOURIER and ODYSSEY trials.
- Video 7 will discuss the impact and safety of early administration of Evolocumab very early after an ACS. Also discussed will be addressed is the question as to which patient groups would be more adequate for EVACS and EVOPACS, treatment.
- Video 8 will discuss the indications, efficacy, and safety of PCSK9i in FH patients.
- Video 9 will discuss ongoing therapeutic developments in the field. Discussion to include : PCSCK9 inhibitors for pediatric patients, ANGPTL3 inhibitors, Lp(a) therapies, and other novel approaches.
The IAS is proud to present this esteemed global faculty. More information on each faculty member can be found here.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
IAS planners and reviewers disclosed no financial relationship.
Disclaimer
This course is designed solely to provide the healthcare professional with information to assist in his/her practice and professional development and is not to be considered a diagnostic tool to replace professional advice or treatment. The course serves as a general guide to the healthcare professional, and therefore, cannot be considered as giving legal, nursing, medical, or other professional advice in specific cases. AKH Inc. specifically disclaim responsibility for any adverse consequences resulting directly or indirectly from information in the course, for undetected error, or through participant’s misunderstanding of the content.
Disclosure of Unlabeled Use and Investigational Product
This educational activity may include discussion of uses of agents that are investigational and/or unapproved by the FDA. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.

The IAS is very grateful to Amgen for their support of this educational activity.
The IAS gratefully acknowledges and thanks the following people for their expertise and help in the translations of the subtitles:
Arabic: Mutaz Al-Khnifsawi (Iraq-Egypt)
Chinese: Chak Fun Law (Macau, China) and Ut Sam Lao (Macau, China)
Portuguese: Marcio Miname (Brasil)
Spanish: Rodrigo Alonso Karlezi (Chile)
 You can also use CardioMedica (an Amazon Alexa skill) to take this Activity
You can also use CardioMedica (an Amazon Alexa skill) to take this Activity
CardioMedica is an education and information channel created for the IAS as an additional way to take education programs using the popular Amazon Alexa device. The programs, offered in English, can be accessed on any Alexa (device or app) using the instructions below.
- First time users: Enable the Skill by saying “Alexa Enable Cardio Medica”
- Once Enabled or Returning Established users: Say , “Alexa Start Cardio Medica“
Tip-After starting the skill the programs will play in order, however, you can also select specific sections from the list below. When Alexa asks you to say “Begin”, just say the catalog number such as “L D L 3”
For more assistance, visit the CardioMedica page on the AudioEducate(click this link) website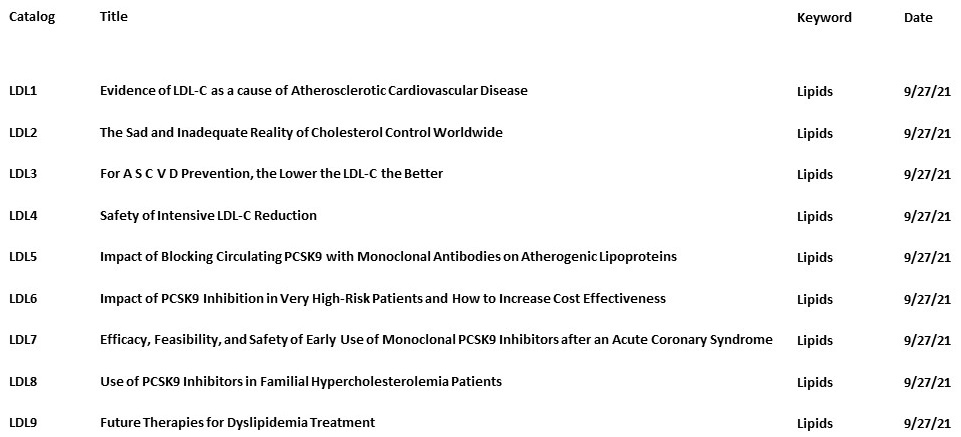


Part 4: Round table with the experts: Where do we stand in our battle against the ravages of diabetes?

Part 3 – Session A: A Hierarchy of Treatment Approaches based on Severity for the T2DM Patient with ASCVD















